AGLE-102TM is a novel therapy manufactured as a composite of human bone marrow mesenchymal stem cell (MSC)-derived extracellular vesicles (EVs).
Extracellular Vesicles
Membrane-bound nanoparticles secreted by cells that carry the key bioactive, paracrine components of MSCs
EVs are considered the key bioactive paracrine mediators of MSCs and carry various proteins and nucleic acids (microRNAs and messenger RNAs among others) with a wide array of pro-regenerative functional impacts on recipient cells. Through paracrine signaling, active proteins and nucleic acids are released from EVs to modulate cell proliferation, migration, and survival of other cells, and can act both locally and distally within the body. Additionally, BM-MSC EVs can transfer critical skin basement membrane proteins, such as collagen IV and collagen VII, which are clinically important for Aegle’s first dermatological indication (McBride et al. 2018).
MSCs are adult stem cells that can be isolated from multiple sources, including human bone marrow (BM). It has been well-established in the literature that BM-MSCs are multipotent stem cells with the potential to differentiate into mesodermal-derived tissues and endogenous stem cell populations. BM-MSCs have demonstrated great potential to promote cutaneous regeneration via their secretion of EVs.
Microvesicles bud directly from the cell’s plasma membrane and exosomes bud into MVBs and are released by fusion of the MVBs with the plasma membrane
Important carriers of MSC-derived nucleic acids, lipids and proteins, including collagen IV and collagen VII
Transmit the regenerative, immunomodulatory, and anti-inflammatory properties of MSCs
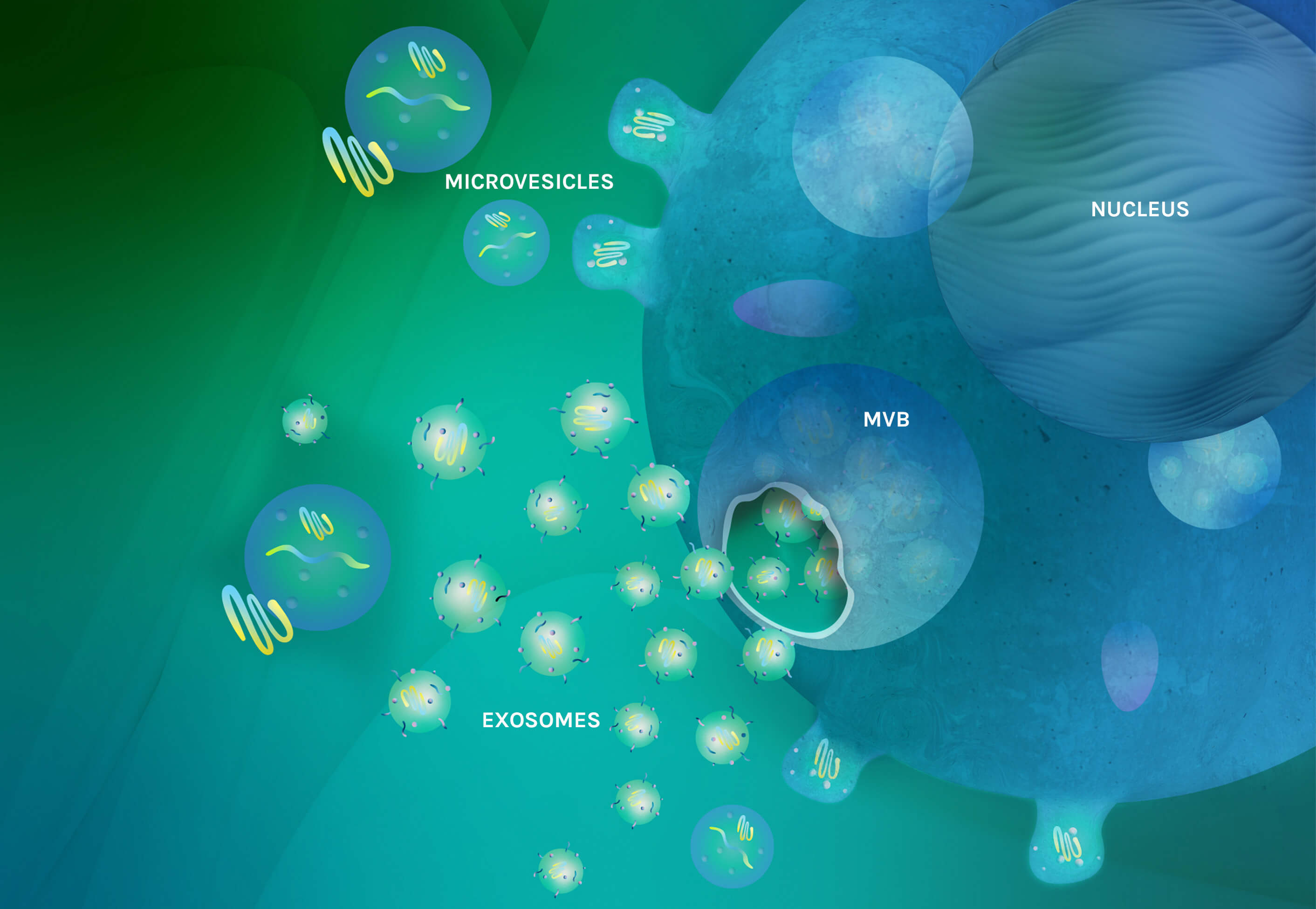
A depiction of a mesenchymal stem cell (MSC) secreting extracellular vesicles (EVs). EVs are a collective of exosomes, microvesicles and apoptotic bodies. Exosomes are formed inside multivesicular bodies (MVB) in the cell and then released into the intracellular space. Microvesicles are derived through outward budding and fission from the plasma membrane. The EVs contain complex materials including nucleic acids, proteins, polypeptides and bioactive lipids.
Aegle Technology
Harnessing the regenerative, immunomodulatory & anti-inflammatory potential of MSC-derived EVs
There are three main subtypes of EVs–exosomes, microvesicles and apoptotic bodies—which are differentiated based on their biogenesis, size, content, and function. The potential of EVs is believed to arise from their action as a collective, rather than through the action of an individual subtype. AGLE-102TM delivers a composite of BM-MSC-derived EVs and is designed to deliver the therapeutically active biomolecules of their parent stem cells.
Advancement of EV therapy has historically been hampered by an inability to isolate EVs in useful quantities while preserving their structural and functional integrity. Aegle’s preclinical research in porcine models has shown that traditional methods of EV isolation can cause structural or functional changes to EVs that can trigger inflammatory responses that interfere with tissue regeneration and healing. In contrast, Aegle’s proprietary harvesting technology yields high-quality vesicles that avoid triggering this inflammatory response and have been shown to promote accelerated healing, minimize or eliminate scarring, promote skin re-pigmentation and hair growth, and stimulate blood vessel and nerve regeneration in a porcine model. Our manufacturing process for AGLE-102 has been scaled for clinical production using allogeneic bone marrow derived MSCs.
EV Therapy
Benefits over stem cell therapy
Research supports the concept that EVs are the mechanism of action of stem cells (McVey et al. Extracellular vesicles; Am J Physiol 316: 1977-1989, 2019). Like stem cells, EVs stimulate and enhance neuronal regeneration, cellular proliferation, cellular migration, and functional regeneration and organization of complex tissue structures in vivo. EVs have also been shown to deliver missing genetic material to diseased or damaged cells, correcting the functionality of the cells. In addition, since EV therapy is a cell-free therapy, there is no risk associated with stem cell migration, differentiation, or engraftment. Additionally, EVs do not require oxygen and can survive in harsh biological environments such as deep abrasions or the GI tract. As such, EVs potentially offer a more robust, safer alternative to MSCs, addressing some of the more complex issues surrounding stem cell therapy.
Publication Spotlight
A proof-of-concept study: EVs as the mechanism of action of MSCs
MSC-derived EVs were hypothesized to be the mechanism of action for the regenerative wound healing demonstrated in a recent clinical study conducted at the University of Miami. As part of this study, burn wound patients experienced rapid healing, little to no scarring, skin re-pigmentation, hair growth and skin elasticity following treatment with cryopreserved MSCs. The MSC dose used in this trial was analyzed prior to patient administration and found to contain billions of EVs that had been secreted from the cells during dose preparation. MSCs do not survive long in a wound (ie they need oxygen) and they have been shown to migrate away from the site of administration. It was hypothesized that EVs were the mediators of the complex, long term wound healing. This trial provided initial clinical proof-of-concept that EVs are the mechanism of action of their parent MSCs.
(Schulman et al. The Effect of MSCs Improves the Healing of Burn Wounds; Scars Burns & Healing Vol 8:1-25, 2022)