Aegle Therapeutics is developing novel therapies using extracellular vesicles (EVs) isolated from mesenchymal stem cells (MSCs) to deliver therapeutic biomolecules to diseased and damaged cells to treat disorders with significant unmet medical need.
EVs are the body’s courier service. MSCs, like all cells, package cell specific cargo into membrane bound EVs and then release the EVs. MSC-EVs travel in the intracellular space or in the bloodstream to recipient cells, delivering contents such as nucleic acids, proteins and immunomodulatory agents. These EVs naturally and efficiently transfer their cargo to target cells and are capable of altering cell function and/or reprogramming cells. They have also been shown to be involved in cell-to-cell communication. Aegle’s patented EV harvesting technology, exclusively licensed from the University of Miami, allows us to gently isolate EVs from MSCs without damaging or modifying the EVs. Aegle’s composite of MSC-EVs has the potential to treat a broad range of indications in multiple therapeutic areas, including dermatology, immunology-based diseases, protein deficient disorders and many others.
The lead indication for our product AGLE-102TM is dystrophic epidermolysis bullosa (DEB), a rare, pediatric disease resulting in severe skin blistering from minor friction, which causes disfigurement, extraordinary pain, and a shortened life span. AGLE-102 provides a unique, multifaceted approach to the treatment of this monogenetic disease in which patients cannot make the protein collagen VII (COL7). Data from preclinical studies demonstrated that AGLE-102 induces recessive DEB fibroblasts (deficient in COL7) to produce COL7. In addition, AGLE-102 has the potential to deliver COL7 protein as well as other cargo important for the functional regeneration and organization of complex tissue structures that may reduce inflammation, accelerate/enhance healing, reduce scarring, and improve overall cosmesis in DEB patients.
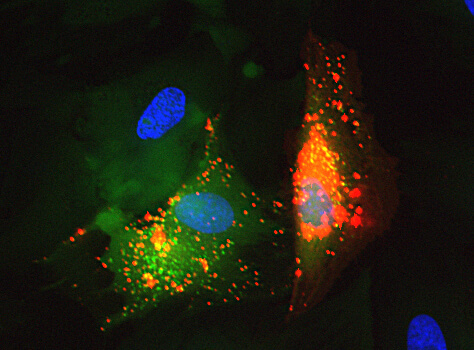
Mesenchymal stem cell (red) secreting extracellular vesicles (red) which fuse into the neighboring fibroblast (green) to deliver their cargo. Nuclei are in blue.
Management Team
Shelley A. Hartman
CEO

Shelley Hartman is a co-founder of Aegle Therapeutics and became Chief Executive Officer in 2019. Prior to Aegle, Ms. Hartman was the Chairman and CEO of LifeSync Corporation, a start-up medical device company. LifeSync was spun out of GMP Companies, Inc. where Ms. Hartman served as the CEO. GMP was founded by investors including TPG, Medtronic and P&G Pharma to advance early-stage biopharmaceutical and medical device product development. Before joining GMP, Ms. Hartman was an investment banker for 19 years, the last seven as a Managing Director at Goldman, Sachs & Co., servicing healthcare/life science clients. Ms. Hartman holds a B.A. from Wellesley College.
Evangelos V. Badiavas, Ph.D., M.D.
Inventor, Founder, Chief Scientific Officer

Dr. Evangelos Badiavas—a board certified dermatologist and dermatopathologist—has led research on wound healing, bone marrow, stem cells, bioengineered skin, dermatology, and translational research for more than 30 years. Dr. Badiavas has particular interests in the histologic evaluation of wounds and has been working with bioengineered skin materials for more than 25 years. Dr. Badiavas was among the first to describe the trafficking and engraftment of bone marrow cells to wounded skin, and the restoration of skin structures by bone marrow stem cells. Dr. Badiavas has been the principal investigator on protocols delivering stem cells to wounds for more than 15 years and was the first to describe the delivery of bone marrow stem cells to chronic wounds in humans. Dr. Badiavas has been awarded three INDs by the FDA for the delivery of stem cells to patients.
Dr. Badiavas’ expertise in bone marrow stem cells began as a graduate student when he worked on erythroid progenitors under the direction of Alan Erslev, Jaimie Caro and Hideko Kaji. Dr. Badiavas has published and presented his findings in several preclinical studies for wounds including rodent and pig models. Dr. Badiavas has also served as a consultant, NIH study section member, and moderator for wound based and stem cell projects.
Heidi Kempinski
Chief Operational Officer

Heidi Kempinski joined Aegle in 2020 as the Senior Vice President and Head of Operations. Ms. Kempinski is a seasoned operations executive who brings over 30 years of experience in global drug development, with core experience in strategic planning, product development, program management and R&D and business operations. Prior to joining Aegle, Ms. Kempinski served as VP of Business Operations and Strategic Alliances at GSK after their acquisition of TESARO, where she led the overall integration of TESARO into GSK. At TESARO, Ms. Kempinski was part of the founding team building R&D operations in parallel to in-licensing and operationalization of the company’s first assets. Ms. Kempinski established pharmaceutical development and manufacturing, quality, regulatory, clinical operations, and program management for the company. Ms. Kempinski also managed all global development and commercialization relationships with licensors and licensees, and collaborators/partners for discovery, clinical development, and diagnostics.
Prior to TESARO, Ms. Kempinski held executive roles in R&D Operations and Program Management at several biopharmaceutical companies including Abraxis BioScience, Eisai, MGI Pharma, Altus Pharmaceuticals, ZYCOS, and Transkaryotic Therapies. Ms. Kempinski earned a BS in microbiology and chemistry from Quinnipiac University and an MBA with a specialty in innovation and entrepreneurship from Northeastern University.
Kathleen Murphy
Head of Clinical Operations

Kat Murphy has over 25 years of experience in the Biotech and Pharmaceutical industries, from both CRO and small biotech perspectives. Kat has led the successful completion of many clinical trials that span multiple indications and phases. Her most recent experience was at Arcturus Therapeutics where she was integral in leading the pivotal trial in Vietnam that led to the first ever approval of a Self-Amplifying RNA (SA-RNA) in the world. Prior to Arcturus, Kat ran an International Phase 3 Lupus study (monoclonal antibody). She also managed the PRESERVE-1 study focused on Preterm Preeclampsia subjects, which is the largest Preeclampsia trial run in the US to date. Kat holds an MSc in Regulatory Affairs from Northeastern University and a B.S. in Biology from Keene State College.
Board of Directors
Scott Braunstein, M.D., Chairman
Scott Braunstein, M.D., brings over 30 years of knowledge and experience from diverse biotechnology and pharmaceutical industry vantage points. He has been an operating partner at Aisling Capital since 2015. Until March 2025, Dr. Braunstein served as President and Chief Executive Officer as well as board member of Marinus Pharmaceuticals, Inc., a neurology therapeutics company acquired by Immedica Pharma AB. Prior to that, he served as Senior Vice President, Strategy and Chief Operating Officer at Pacira Pharmaceuticals, Inc., a specialty pharmaceutical company. Prior to Pacira, Dr. Braunstein spent 12 years with J.P. Morgan Asset Management as a Healthcare Analyst and Managing Director and as Portfolio Manager of the JP Morgan Global Healthcare Fund. He currently serves on the board of directors for Atai Life Sciences N.V., a clinical-stage biopharmaceutical company, Caribou Biosciences, Inc., a clinical-stage CRISPR genome-editing biopharmaceutical company, RAPT Therapeutics, Inc. (Nasdaq: RAPT), and Site One Therapeutics, a clinical-stage neurology company that is being acquired by Eli Lilly. Dr. Braunstein previously served on the board of directors of Trevena Inc., Esperion Therapeutics, Inc., Ziopharm Oncology Inc., Protara Therapeutics, Inc. (previously known as ArTara Therapeutics, Inc.) and Constellation Pharmaceuticals, Inc. Dr. Braunstein began his career as a practicing physician at the Summit Medical Group and as an Assistant Clinical Professor at Albert Einstein College of Medicine and Columbia University Medical Center. Dr. Braunstein received an M.D. from the Albert Einstein College of Medicine and a BASc in biology from Cornell University.
Evangelos V. Badiavas, Ph.D., M.D.
Dr. Evangelos Badiavas—a board certified dermatologist and dermatopathologist—has led research on wound healing, bone marrow, stem cells, bioengineered skin, dermatology, and translational research for more than 30 years. Dr. Badiavas has particular interests in the histologic evaluation of wounds and has been working with bioengineered skin materials for more than 25 years. Dr. Badiavas was among the first to describe the trafficking and engraftment of bone marrow cells to wounded skin, and the restoration of skin structures by bone marrow stem cells. Dr. Badiavas has been the principal investigator on protocols delivering stem cells to wounds for more than 15 years and was the first to describe the delivery of bone marrow stem cells to chronic wounds in humans. Dr. Badiavas has been awarded three INDs by the FDA for the delivery of stem cells to patients.
Dr. Badiavas’ expertise in bone marrow stem cells began as a graduate student when he worked on erythroid progenitors under the direction of Alan Erslev, Jaimie Caro and Hideko Kaji. Dr. Badiavas has published and presented his findings in several preclinical studies for wounds including rodent and pig models. Dr. Badiavas has also served as a consultant, NIH study section member, and moderator for wound based and stem cell projects.
Shelley A. Hartman
Shelley Hartman is a co-founder of Aegle Therapeutics and became Chief Executive Officer in 2019. Prior to Aegle, Ms. Hartman was the Chairman and CEO of LifeSync Corporation, a start-up medical device company. LifeSync was spun out of GMP Companies, Inc. where Ms. Hartman served as the CEO. GMP was founded by investors including TPG, Medtronic and P&G Pharma to advance early-stage biopharmaceutical and medical device product development. Before joining GMP, Ms. Hartman was an investment banker for 19 years, the last seven as a Managing Director at Goldman, Sachs & Co., servicing healthcare/life science clients. Ms. Hartman holds a B.A. from Wellesley College.
Lonnie Moulder
Lonnie Moulder joined our Board in January 2020. Mr. Moulder is General Partner of Tellus BioVentures, LLC, a life science investment fund he founded in 2019. Previously Mr. Moulder was the co-founder, Chief Executive Officer and a member of the board of directors of TESARO, Inc., a public oncology-focused biopharmaceutical company that was acquired by GlaxoSmithKline plc. Previously Mr. Moulder served as Vice Chairman of the board of directors, President and Chief Executive Officer of Abraxis BioScience, Inc., a biotechnology company. Before that, Mr. Moulder served as Vice Chairman of Eisai Corporation of North America, a pharmaceutical company and wholly owned subsidiary of Eisai Co., Ltd., following Eisai Co., Ltd.’s acquisition of MGI PHARMA, Inc. Mr. Moulder served as President and Chief Executive Officer and as a member of the board of directors of MGI PHARMA, Inc.
Mr. Moulder is a Trustee of Temple University and serves as a board director for several public and privately held biotechnology companies. Mr. Moulder earned a B.S. in pharmacy from Temple University and a M.B.A. from the University of Chicago.
Steve O'Hara
After earning an AB and MBA from Harvard University, Steve O'Hara began his career in marketing at Procter & Gamble. Moving into general management, Steve held C-suite positions as President of Carroll Reed, President of Specialty Catalog, Chairman and CEO of Rawlings Sporting Goods (NASDAQ:RAWL) and CEO of Angelica Corporation (NYSE:AGL). After retiring in 2011, Steve began a second career as an independent board member and angel investor. He joined New World Angels (NWA) in 2012. In 2016, Steve became President of NWA. After 6 years as President, Steve became Chairman of NWA in February, 2022. NWA now boasts 109 members throughout the state of Florida.
David Schimel, M.D.
Dr. David Schimel joined our Board in January of 2020. Dr. Schimel practiced internal medicine and endocrinology in the Chicago suburbs and was the cofounder and managing partner of Deerpath Medical Associates, a 30-physician multispecialty group. Dr. Schimel also served as the Director of Midwest Clinical Research Associates and both supervised and participated in a number of Phase 3 and 4 clinical trials in various medical disciplines. After receiving an MBA degree in 1996, Dr. Schimel founded International Equity Trading Partners and managed assets for institutional clients based on a proprietary computer algorithm that predicted the short-term fluctuations of equity and interest rate markets. Dr. Schimel earned a B.S. degree from the University of Illinois, a M.D. degree at the University of Michigan and a M.B.A. from the Kellogg School of Management at Northwestern University.
Mike Tanji
Mike Tanji is a Director of Alliance Forum Foundation, Director of DEFTA Capital Inc. and General Manager of DEFTA Healthcare Technologies, L.P. Mr. Tanji joined the Alliance Forum Foundation in 2009 and has been in his current position since 2017. Mr. Tanji started his career at The Long-Term Credit Bank of Japan, Ltd. (LTCB) followed by a secondment to the Ministry of International Trade and Industry. After working in the United States at a subsidiary of LTCB, Mr. Tanji managed the interest rate policy and portfolio management at Business Planning Division, then supported corporate customer entry to Asian countries at Asian Division as Associate General Manager of both organizations. Since 1995, when he left LTCB, Mr. Tanji served as Senior Consultant at Jomon Associates, Executive Assistant to the President at Shin Ginko Tokyo Co., Ltd., and Senior Managing Executive Officer at Aruze Corporation. Mr. Tanji is also a Juris Doctor and a Director of Japan Initiative.